On Friday a San Diego-based biotech company ViaCyte filed an Investigational New Drug (IND) application with the FDA to proceed with Phase I/II clinical trials in people with type 1 diabetes. The trial will evaluate the safety and efficacy of a new stem cell-derived encapsulated cell replacement therapy, known officially as VC-O1. Basically, that product uses pancreas endoderm cells derived from embryonic stem cells and would be put into the body using something called the Encaptra delivery system.
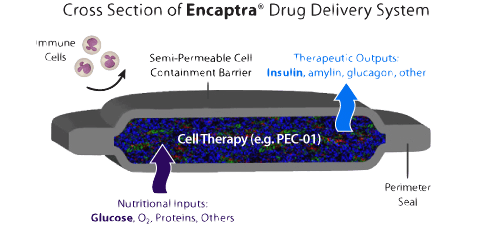
Encaptra is a flat device about the width of a credit card. It is implanted in under the skin (possibly in the upper back) through a simple outpatient surgical procedure and designed to last at least a year, and possibly up to five years before it is used and requires replacement.
This device would be loaded with insulin-producing cells before implantation, and contains pores that allow glucose and insulin to be transferred through, but not antibodies — meaning insulin would be released as needed in response to the varying glucose levels, but no immuno-suppression drugs will be necessary because the device is protected from autoimmune attack by the sheet’s membrane.
Officially known VC-01-Encaptra if approved will revolutionise insulin delivery making it easier to administer therapy to diabetic patients and will also increase complaince. It is expected that the delivery system may be available for commercial use in the next ten years.
To view how Diabetes Mine reported this story click here.
Source Diabetes Mine