 The FDA has approved a new inhaled insulin “Afrezza” which is hoped will improve glycemic control in both type 1 and type 2 diabetes. This is a new option for delivering insulin and will be prescribed by doctors for use at mealtime and will be used as a part of the overall strategy in controlling sugar levels in any given patent.
The FDA has approved a new inhaled insulin “Afrezza” which is hoped will improve glycemic control in both type 1 and type 2 diabetes. This is a new option for delivering insulin and will be prescribed by doctors for use at mealtime and will be used as a part of the overall strategy in controlling sugar levels in any given patent.
The drug will also come with the most serious warning available. The warning will state that that acute bronchospasm has been seen in patients with asthma and chronic obstructive pulmonary disease and that it should not be used in patients with those conditions.
Afrezza is a rapid-acting inhaled insulin to be taken before meals or within 20 minutes of starting a meal. Afrezza is not a substitute for long-acting insulin and must be used in combination with long-acting insulin in patients with type 1 diabetes, however in certain type 2 patients it may be the only insulin that will be required. It is not recommended for the treatment of diabetic induced ketoacidosis or in patients who have chronic lung disease or who smoke.
Efficacy and safety data came from studies involving a total of 3,017 patients, including 1,026 with type 1 and 1,991 with type 2 diabetes. At 24 weeks, Afrezza reduced HbA1c levels by the prespecified end point of 0.4 percentage points in both groups. HbA1c reduction was inferior to that of injected insulin among type 1 patients but significantly superior to placebo among type 2 patients who were also taking oral glucose-lowering medications.
The most observed side effects associated with Afrezza in clinical trials were hypoglycemia, cough, and throat pain or irritation.
The listed side effects and warning are as follows:
- Low blood sugar (hypoglycemia). Signs and symptoms that may indicate low blood sugar include:
- Dizziness or light-headedness, sweating, confusion, headache, blurred vision, slurred speech, shakiness, fast heartbeat, anxiety, irritability or mood change, hunger.
- Decreased lung function. Your healthcare provider should check how your lungs are working before you start using Afrezza, 6 months after you start using it and yearly after that.
- Lung cancer. In studies of Afrezza in people with diabetes, lung cancer occurred in a few more people who were taking Afrezza than in people who were taking other diabetes medications. There were too few cases to know if lung cancer was related to Afrezza. If you have lung cancer, you and your healthcare provider should decide if you should use Afrezza.
- Diabetic ketoacidosis. Talk to your healthcare provider if you have an illness. Your Afrezza dose or how often you check your blood sugar may need to be changed.
- Severe allergic reaction (whole body reaction). Get medical help right away if you have any of these signs or symptoms of a severe allergic reaction: a rash over your whole body, trouble breathing, a fast heartbeat, or sweating.
- Low potassium in your blood (hypokalemia).
- Heart failure. Taking certain diabetes pills called thiazolidinediones or “TZDs” with Afrezza may cause heart failure in some people. This can happen even if you have never had heart failure or heart problems before. If you already have heart failure it may get worse while you take TZDs with Afrezza. Your healthcare provider should monitor you closely while you are taking TZDs with Afrezza. Tell your healthcare provider if you have any new or worse symptoms of heart failure including:
- Shortness of breath, swelling of your ankles or feet, sudden weight gain. Treatment with TZDs and Afrezza may need to be changed or stopped by your healthcare provider if you have new or worse heart failure.
Afrezza will not replace the need for injected long-acting insulin for the group of patients who need it or who are currently using long acting insulin.
Because it is inhaled, Afrezza is absorbed more quickly, so from the time it’s inhaled to the time it actually peaks [in the blood] is 15 to 20 minutes, while injected insulin may take up to an hour to peak when taken before a meal.
The body also clears Afrezza more quickly than insulin injected at mealtime. Besides its rapid peak, the drug is ”pretty much gone in 2 or 3 hours. The rapid acting injected insulins usually are present for about 4 hours. So Afrezza is fast in and fast out, more like what the pancreas actually does.
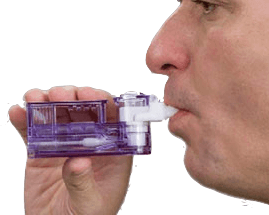
Afrezza will come in a powder form with a small inhaler which is pocket sized and easy to train and use. Different doses come in multiple cartridges which each contain a single dose that is disposable after use.
In a 24-week study, researchers compared Afrezza with a rapid-acting, injected insulin in more than 500 patients with type 1 diabetes. Afrezza and injected insulin controlled blood sugar equally well and Afrezza seems to have less hypoglycemia due to its fast in and fast out cycle.
Due to its also being in the body less time, patients may need less insulin and this could account for less weight gain when compared to injected insulin.
To view the Afrezza Web Site click here
To view the Afrezza medication guide please click here





